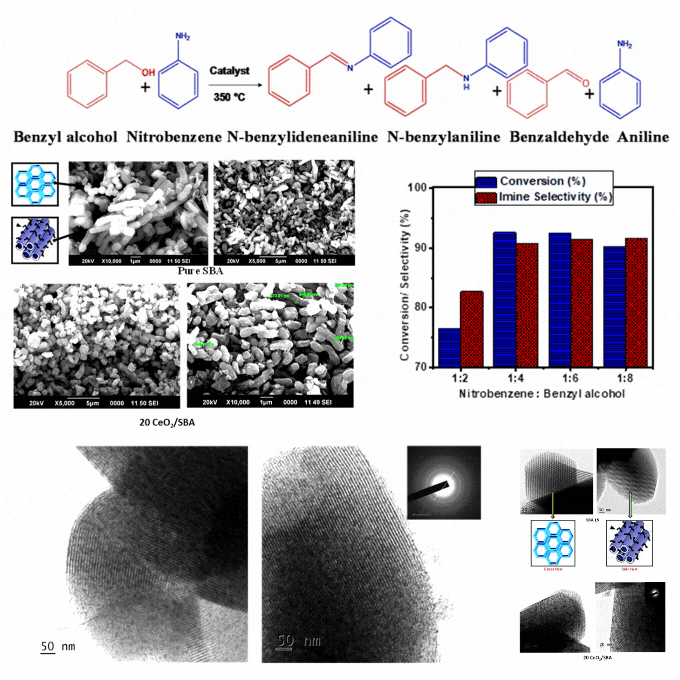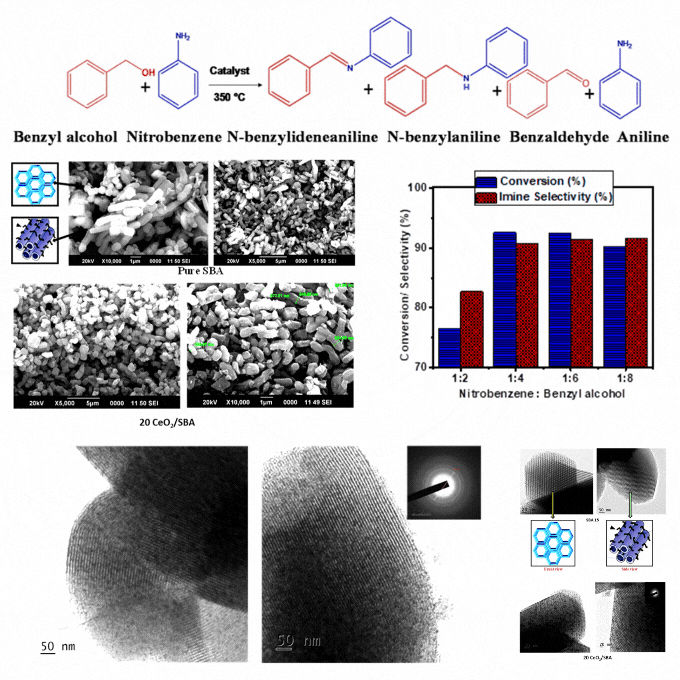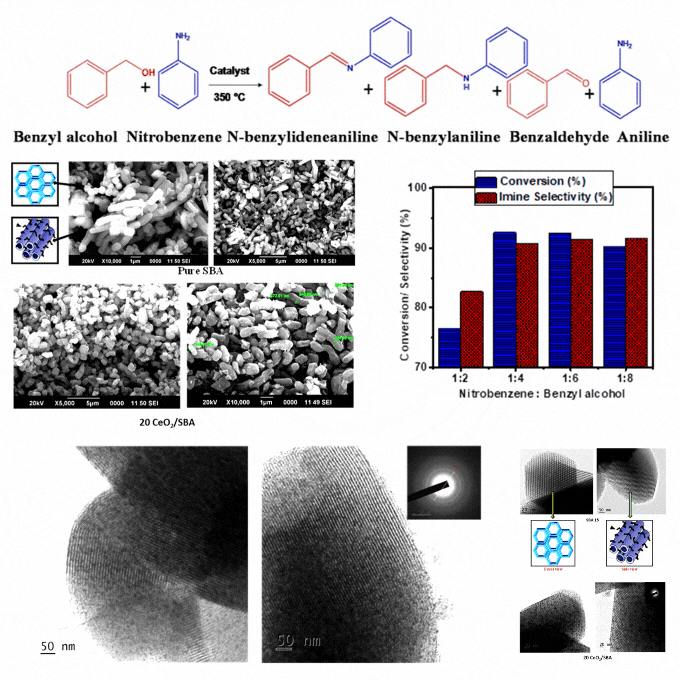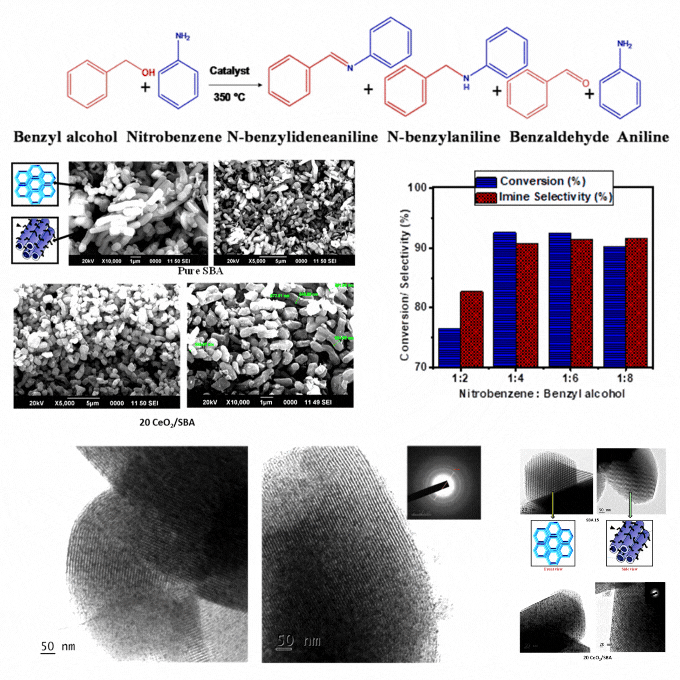
Transformative process: Crafting imines from nitrobenzene and benzyl alcohol coupling with cerium oxide modified mesoporous SBA-15
DOI:
https://doi.org/10.24036/teknomekanik.v7i2.31772Keywords:
mesoporous SBA-15, imine formation, ceria, catalyst developmentAbstract
A low-cost and eco-friendly cerium oxide modified mesoporous SBA-15 catalyst was developed by wet impregnation. It enables sequential oxidation of benzyl alcohol and reduction of nitrobenzene, followed by imine formation in a single and solvent-free system. Characterization confirms homogeneous cerium oxide dispersion, high stability, and enhanced redox properties. The optimized catalyst demonstrates excellent conversion and selectivity, attributed to the mesoporous SBA support, acidic properties, and cerium's redox functionality. Elevated temperatures enhance benzyl alcohol dehydration and hydrogen diffusion, facilitating intermediate aniline formation by a borrowing-hydrogen mechanism and followed by imine synthesis. It eliminates solvents, reduces byproducts, and achieves high atom economy and renewability. It presents a significant advance in sustainable catalysis. The catalyst's robustness and ease of recovery strengthen its practicality for repeated cycles. The findings provide a scalable and energy-efficient solution for greener imine synthesis with potential applications in industrial processes requiring efficient and eco-friendly chemical production. It ensures minimal environmental impact and offers a cost-effective pathway for high-value chemical synthesis. This study supports the SDGs by promoting sustainable industrial practices through the development of a low-cost and eco-friendly catalyst that reduces environmental impact, enhances energy efficiency, and contributes to greener chemical production.
Downloads
References
S. Sobhani, H. Hosseini Moghadam, S. R. Derakhshan, and J. M. Sansano, “Tandem imine formation via auto-hydrogen transfer from alcohols to nitro compounds catalyzed by a nanomagnetically recyclable copper catalyst under solvent-free conditions,” RSC Advances, vol. 11, no. 31, pp. 19121–19127, 2021, https://doi.org/10.1039/D1RA02347K
D. Wang and D. Astruc, “The Golden Age of Transfer Hydrogenation,” Chemical Reviews, vol. 115, no. 13, pp. 6621–6686, Jul. 2015, https://doi.org/10.1021/acs.chemrev.5b00203
H. Sun, F. Su, J. Ni, Y. Cao, H. He, and K. Fan, “Gold Supported on Hydroxyapatite as a Versatile Multifunctional Catalyst for the Direct Tandem Synthesis of Imines and Oximes,” Angewandte Chemie International Edition, vol. 48, no. 24, pp. 4390–4393, Jun. 2009, https://doi.org/10.1002/anie.200900802
S. Kegnæs, J. Mielby, U. V. Mentzel, C. H. Christensen, and A. Riisager, “Formation of imines by selective gold-catalysed aerobic oxidative coupling of alcohols and amines under ambient conditions,” Green Chemistry, vol. 12, no. 8, p. 1437, 2010, https://doi.org/10.1039/c0gc00126k
L. L. Santos, P. Serna, and A. Corma, “Chemoselective Synthesis of Substituted Imines, Secondary Amines, and β‐Amino Carbonyl Compounds from Nitroaromatics through Cascade Reactions on Gold Catalysts,” Chemistry – A European Journal, vol. 15, no. 33, pp. 8196–8203, Aug. 2009, https://doi.org/10.1002/chem.200900884
L. H. Park, E. M. Leitao, and C. C. Weber, “Green imine synthesis from amines using transition metal and micellar catalysis,” Organic & Biomolecular Chemistry, vol. 22, no. 2, pp. 202–227, 2024, https://doi.org/10.1039/D3OB01730C
S. Wu, H. Zhang, Q. Cao, Q. Zhao, and W. Fang, “Efficient imine synthesis via oxidative coupling of alcohols with amines in an air atmosphere using a mesoporous manganese–zirconium solid solution catalyst,” Catalysis Science & Technology, vol. 11, no. 3, pp. 810–822, 2021, https://doi.org/10.1039/D0CY02288H
M. Sankar et al., “Supported bimetallic nano-alloys as highly active catalysts for the one-pot tandem synthesis of imines and secondary amines from nitrobenzene and alcohols,” Catalysis Science & Technology, vol. 6, no. 14, pp. 5473–5482, 2016, https://doi.org/10.1039/C6CY00425C
L. Aschwanden, T. Mallat, F. Krumeich, and A. Baiker, “A simple preparation of an efficient heterogeneous gold catalyst for aerobic amine oxidation,” Journal of Molecular Catalysis A: Chemical, vol. 309, no. 1–2, pp. 57–62, Aug. 2009, https://doi.org/10.1016/j.molcata.2009.04.015
T. Ishida, N. Kawakita, T. Akita, and M. Haruta, “One-potN-alkylation of primary amines to secondary amines by gold clusters supported on porous coordination polymers,” Gold Bull, vol. 42, no. 4, pp. 267–274, Dec. 2009, https://doi.org/10.1007/BF03214948
T. Ishida, R. Takamura, T. Takei, T. Akita, and M. Haruta, “Support effects of metal oxides on gold-catalyzed one-pot N-alkylation of amine with alcohol,” Applied Catalysis A: General, vol. 413–414, pp. 261–266, Jan. 2012, https://doi.org/10.1016/j.apcata.2011.11.017
Q. Peng, Y. Zhang, F. Shi, and Y. Deng, “Fe2O3-supported nano-gold catalyzed one-pot synthesis of N-alkylated anilines from nitroarenes and alcohols,” Chemical Communications, vol. 47, no. 22, p. 6476, 2011, https://doi.org/10.1039/c1cc11057h
E. A. Artiukha et al., “One-pot reductive amination of aldehydes with nitroarenes over an Au/Al 2 O 3 catalyst in a continuous flow reactor,” Catalysis Science & Technology, vol. 5, no. 10, pp. 4741–4745, 2015, https://doi.org/10.1039/C5CY00964B
X. Liu et al., “C-C Cross‐Coupling of Primary and Secondary Benzylic Alcohols Using Supported Gold‐Based Bimetallic Catalysts,” ChemSusChem, vol. 6, no. 4, pp. 604–608, Apr. 2013, https://doi.org/10.1002/cssc.201200804
K. Selvam, H. Sakamoto, Y. Shiraishi, and T. Hirai, “One-pot synthesis of secondary amines from alcohols and nitroarenes on TiO 2 loaded with Pd nanoparticles under UV irradiation,” New Journal of Chemistry, vol. 39, no. 4, pp. 2467–2473, 2015, https://doi.org/10.1039/C4NJ01851F
H. Liu, G. Khuan Chuah, and S. Jaenicke, “Alumina-entrapped Ag catalyzed nitro compounds coupled with alcohols using borrowing hydrogen methodology,” Physical Chemistry Chemical Physics, vol. 17, no. 22, pp. 15012–15018, 2015, https://doi.org/10.1039/C5CP00330J
U. Mandi, A. S. Roy, S. K. Kundu, S. Roy, A. Bhaumik, and Sk. M. Islam, “Mesoporous polyacrylic acid supported silver nanoparticles as an efficient catalyst for reductive coupling of nitrobenzenes and alcohols using glycerol as hydrogen source,” Journal of Colloid and Interface Science, vol. 472, pp. 202–209, Jun. 2016, https://doi.org/10.1016/j.jcis.2016.03.037
W. He et al., “Pt–Sn/γ‐Al 2 O 3 ‐Catalyzed Highly Efficient Direct Synthesis of Secondary and Tertiary Amines and Imines,” Chemistry – A European Journal, vol. 17, no. 47, pp. 13308–13317, Nov. 2011, https://doi.org/10.1002/chem.201101725
C.-C. Lee and S.-T. Liu, “Preparation of secondary and tertiary amines from nitroarenes and alcohols,” Chemical Communications, vol. 47, no. 24, p. 6981, 2011, https://doi.org/10.1039/c1cc11609f
J. M. Pérez, R. Cano, M. Yus, and D. J. Ramón, “Straightforward Synthesis of Aromatic Imines from Alcohols and Amines or Nitroarenes Using an Impregnated Copper Catalyst,” European Journal of Organic Chemistry, vol. 2012, no. 24, pp. 4548–4554, Aug. 2012, https://doi.org/10.1002/ejoc.201200319
S. Z. Anbardan, J. Mokhtari, A. Yari, and A. H. Bozcheloei, “Direct synthesis of amides and imines by dehydrogenative homo or cross-coupling of amines and alcohols catalyzed by Cu-MOF,” RSC Advances, vol. 11, no. 34, pp. 20788–20793, 2021, https://doi.org/10.1039/D1RA03142B
B. D. Bankar, K. Ravi, S. Subramanian, and A. V. Biradar, “Niobium Oxide Supported on Cubic Spinel Cobalt Oxide as an Efficient Heterogeneous Catalyst for the Synthesis of Imines via Dehydrogenative Coupling of Amines and Alcohols,” Catal Letters, vol. 152, no. 12, pp. 3733–3746, Dec. 2022, https://doi.org/10.1007/s10562-022-03943-2
Y. Zheng et al., “One Pot Synthesis of Imines from Aromatic Nitro Compounds with a Novel Ni/SiO2 Magnetic Catalyst,” Catal Letters, vol. 128, no. 3–4, pp. 465–474, Mar. 2009, https://doi.org/10.1007/s10562-008-9774-0
A. Zanardi, J. A. Mata, and E. Peris, “One‐Pot Preparation of Imines from Nitroarenes by a Tandem Process with an Ir–Pd Heterodimetallic Catalyst,” Chemistry – A European Journal, vol. 16, no. 34, pp. 10502–10506, Sep. 2010, https://doi.org/10.1002/chem.201000801
J.-M. Huang, J.-F. Zhang, Y. Dong, and W. Gong, “An Effective Method To Prepare Imines from Aldehyde, Bromide/Epoxide, and Aqueous Ammonia,” The Journal of Organic Chemistry, vol. 76, no. 9, pp. 3511–3514, May 2011, https://doi.org/10.1021/jo102455q
J. Li et al., “Interfacial Reaction-Directed Green Synthesis of CeO 2 –MnO 2 Catalysts for Imine Production through Oxidative Coupling of Alcohols and Amines,” Inorganic Chemistry, vol. 62, no. 8, pp. 3692–3702, Feb. 2023, https://doi.org/10.1021/acs.inorgchem.3c00095
R. Lima Oliveira, K. A. Ledwa, O. Chernyayeva, S. Praetz, C. Schlesiger, and L. Kepinski, “Cerium Oxide Nanoparticles Confined in Doped Mesoporous Carbons: A Strategy to Produce Catalysts for Imine Synthesis,” Inorganic Chemistry, vol. 62, no. 33, pp. 13554–13565, Aug. 2023, https://doi.org/10.1021/acs.inorgchem.3c01985
S. Parambadath and A. P. Singh, “Ru(II)-Chiral (1R,2S)-(+)-cis-1-amino-2-indanol immobilized over SBA-15 for asymmetric transfer hydrogenation reaction of prochiral ketones,” Catal Today, vol. 141, no. 1–2, pp. 161–167, Mar. 2009, https://doi.org/10.1016/j.cattod.2008.04.003
C. Veranitisagul et al., “Novel Recovery of Nano-Structured Ceria (CeO2) from Ce(III)-Benzoxazine Dimer Complexes via Thermal Decomposition,” International Journal of Molecular Sciences, vol. 12, no. 7, pp. 4365–4377, Jul. 2011, https://doi.org/10.3390/ijms12074365
Z. Mu, J. J. Li, H. Tian, Z. P. Hao, and S. Z. Qiao, “Synthesis of mesoporous Co/Ce-SBA-15 materials and their catalytic performance in the catalytic oxidation of benzene,” Materials Research Bulletin, vol. 43, no. 10, pp. 2599–2606, Oct. 2008, https://doi.org/10.1016/j.materresbull.2007.10.037
K. S. W. Sing, “Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984),” Pure and Applied Chemistry, vol. 57, no. 4, pp. 603–619, Jan. 1985, https://doi.org/10.1351/pac198557040603
C. L. Peza-Ledesma, L. Escamilla-Perea, R. Nava, B. Pawelec, and J. L. G. Fierro, “Supported gold catalysts in SBA-15 modified with TiO2 for oxidation of carbon monoxide,” Applied Catalysis A: General, vol. 375, no. 1, pp. 37–48, Feb. 2010, https://doi.org/10.1016/j.apcata.2009.12.009
X. Li, S. Huang, Q. Xu, and Y. Yang, “Preparation of WO3-SBA-15 mesoporous molecular sieve and its performance as an oxidative desulfurization catalyst,” Transition Metal Chemistry, vol. 34, no. 8, pp. 943–947, Nov. 2009, https://doi.org/10.1007/s11243-009-9285-x
T. Tsoncheva et al., “Catalytic VOCs elimination over copper and cerium oxide modified mesoporous SBA-15 silica,” Applied Catalysis A: General, vol. 453, pp. 1–12, Feb. 2013, https://doi.org/10.1016/j.apcata.2012.12.007
S. C. Laha, P. Mukherjee, S. R. Sainkar, and R. Kumar, “Cerium Containing MCM-41-Type Mesoporous Materials and their Acidic and Redox Catalytic Properties,” Journal of Catalysis, vol. 207, no. 2, pp. 213–223, Apr. 2002, https://doi.org/10.1006/jcat.2002.3516
B. M. Reddy et al., “Structural Characterization and Catalytic Activity of Nanosized Ce x M 1- x O 2 (M = Zr and Hf) Mixed Oxides,” The Journal of Physical Chemistry C, vol. 112, no. 31, pp. 11729–11737, Aug. 2008, https://doi.org/10.1021/jp802674m
D. Zhao et al., “Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores,” Science (1979), vol. 279, no. 5350, pp. 548–552, Jan. 1998, https://doi.org/10.1126/science.279.5350.548
J. Li, Y. Hao, H. Li, M. Xia, X. Sun, and L. Wang, “Direct synthesis of CeO2/SiO2 mesostructured composite materials via sol–gel process,” Microporous and Mesoporous Materials, vol. 120, no. 3, pp. 421–425, Apr. 2009, https://doi.org/10.1016/j.micromeso.2008.12.014
H. Zhang et al., “Synthesis, characterization, and catalytic performance of copper-containing SBA-15 in the phenol hydroxylation,” Journal of Colloid and Interface Science, vol. 380, no. 1, pp. 16–24, Aug. 2012, https://doi.org/10.1016/j.jcis.2012.04.059
X.-P. Fu, H. Zhao, and C.-J. Jia, “Ceria-based supported metal catalysts for the low-temperature water–gas shift reaction,” Chemical Communications, vol. 60, no. 98, pp. 14537–14556, 2024, https://doi.org/10.1039/D4CC04072D
J. Shen and C. Hess, “Controlling the dispersion of ceria using nanoconfinement: application to CeO 2 /SBA-15 catalysts for NH 3 -SCR,” Materials Advances, vol. 2, no. 22, pp. 7400–7412, 2021, https://doi.org/10.1039/D1MA00658D
E. A. Artiukha et al., “One-pot reductive amination of aldehydes with nitroarenes over an Au/Al 2 O 3 catalyst in a continuous flow reactor,” Catalysis Science & Technology, vol. 5, no. 10, pp. 4741–4745, 2015, https://doi.org/10.1039/C5CY00964B
C. Tang, L. He, Y. Liu, Y. Cao, H. He, and K. Fan, “Direct One‐Pot Reductive N‐Alkylation of Nitroarenes by using Alcohols with Supported Gold Catalysts,” Chemistry – A European Journal, vol. 17, no. 26, pp. 7172–7177, Jun. 2011, https://doi.org/10.1002/chem.201100393
K. Selvam, H. Sakamoto, Y. Shiraishi, and T. Hirai, “One-pot synthesis of secondary amines from alcohols and nitroarenes on TiO 2 loaded with Pd nanoparticles under UV irradiation,” New Journal of Chemistry, vol. 39, no. 4, pp. 2467–2473, 2015, https://doi.org/10.1039/C4NJ01851F
C.-C. Lee and S.-T. Liu, “Preparation of secondary and tertiary amines from nitroarenes and alcohols,” Chemical Communications, vol. 47, no. 24, p. 6981, 2011, https://doi.org/10.1039/c1cc11609f
J. M. Pérez, R. Cano, M. Yus, and D. J. Ramón, “Straightforward Synthesis of Aromatic Imines from Alcohols and Amines or Nitroarenes Using an Impregnated Copper Catalyst,” European Journal of Organic Chemistry, vol. 2012, no. 24, pp. 4548–4554, Aug. 2012, https://doi.org/10.1002/ejoc.201200319
Y. Zheng et al., “One Pot Synthesis of Imines from Aromatic Nitro Compounds with a Novel Ni/SiO2 Magnetic Catalyst,” Catal Letters, vol. 128, no. 3–4, pp. 465–474, Mar. 2009, https://doi.org/10.1007/s10562-008-9774-0
P. Fristrup, M. Tursky, and R. Madsen, “Mechanistic investigation of the iridium-catalysed alkylation of amines with alcohols,” Organic & Biomolecular Chemistry, vol. 10, no. 13, p. 2569, 2012, https://doi.org/10.1039/c2ob06603c
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Soumini Chandralayam, Sugunan Sankaran

This work is licensed under a Creative Commons Attribution 4.0 International License.