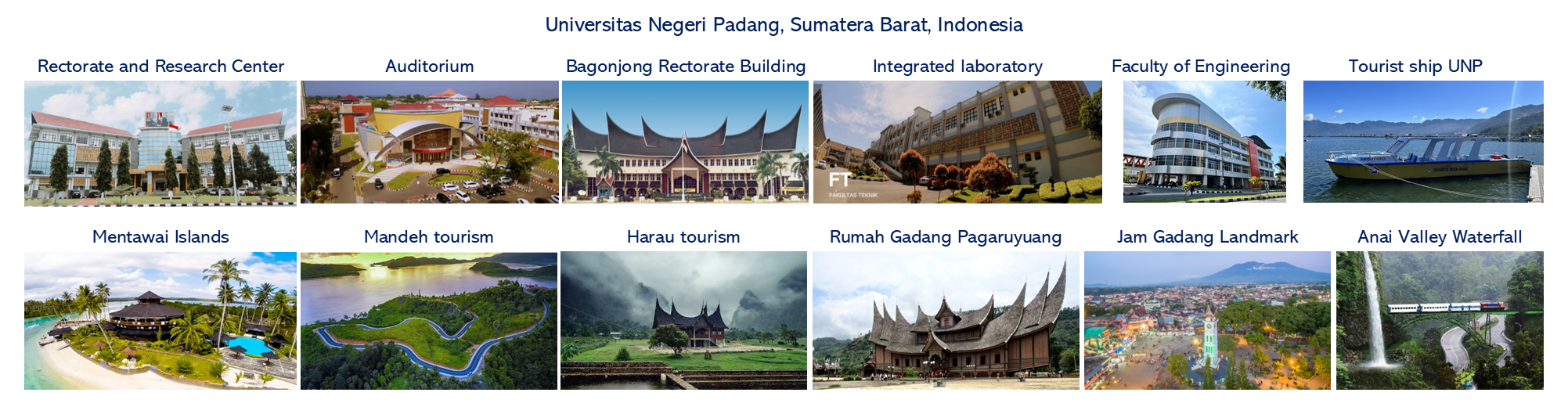
High-pressure adsorption isothermal on a novel microporous material from polyethylene terephthalate plastic waste in carbon dioxide capture applications
DOI:
https://doi.org/10.24036/teknomekanik.v8i1.36172Keywords:
carbon capture, adsorption isotherm, heat of adsorption, isosteric adsorptionAbstract
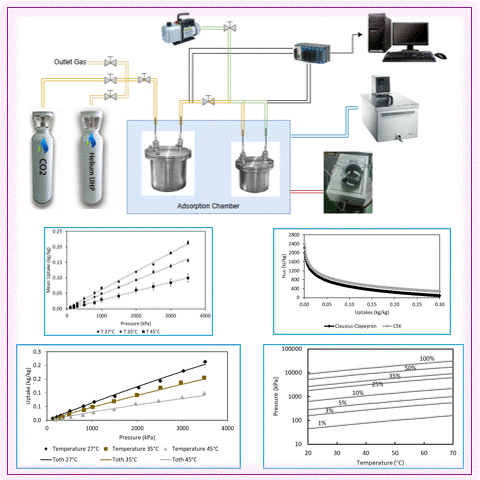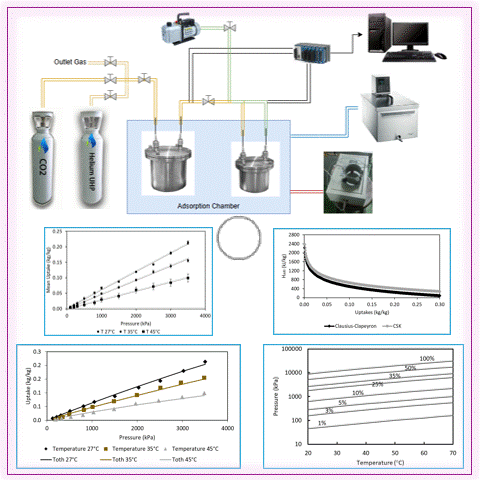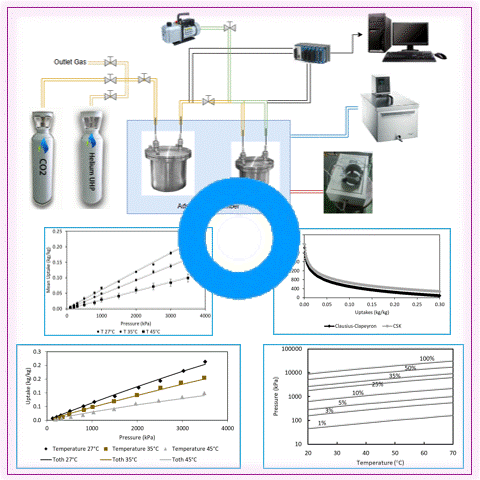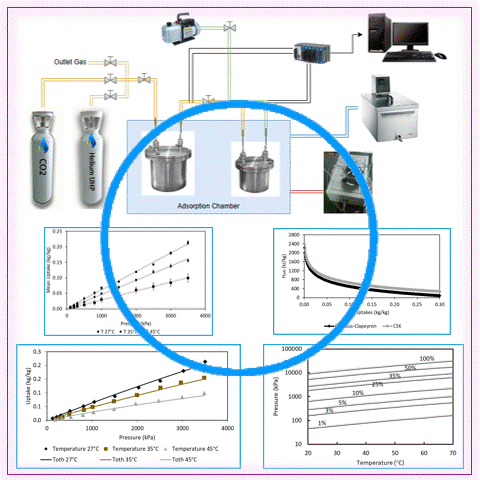
Carbon capture is a vital strategy for mitigating climate change by reducing industrial CO2 emissions. Adsorption technology using microporous material shows significant promise. However, significant challenges persist in developing cost-effective and sustainable adsorbents. This study addresses this issue by simultaneously enabling CO2 adsorption and plastic waste utilization through activated carbon derived from polyethylene terephthalate (PET). It was evaluated under isothermal conditions (27°C, 35°C, and 45°C) at pressures up to 3500 kPa. The maximum CO2 adsorption capacity was 0.21313 kg/kg at 27°C and 3504.39 kPa, demonstrating the effectiveness of PET-derived activated carbon in capturing CO2. The Toth isotherm model exhibited a strong fit with experimental data, with an R2 of more than 99%. The Clausius-Clapeyron equation yielded an adsorption heat of 2223.66 kJ/kg using the Toth fitting, and the Chakraborty-Saha-Koyama model yielded a heat of 2383.65 kJ/kg, confirming strong adsorption potential. These results underline PET waste as a viable precursor for sustainable carbon capture adsorbents. Furthermore, the results provide essential data for developing numerical models to optimize adsorption-based carbon capture technologies.
Downloads
References
W. Suksuwan and M. Wae-hayee, “Enhancing Combustion Efficiency in Combustion Chamber: A Comparative Study of Single and Double Tangential Inlet Configurations,” Journal of Advanced Research in Numerical Heat Transfer, vol. 16, no. 1, pp. 70–81, 2024, https://doi.org/10.37934/arnht.16.1.7081
H. Van Asselt and F. Green, “COP26 and the dynamics of anti-fossil fuel norms,” Wiley Interdiscip Rev Clim Change, vol. 14, no. 3, pp. 1–12, 2023, https://doi.org/10.1002/wcc.816
T. T. Nguyen, U. Grote, F. Neubacher, D. B. Rahut, M. H. Do, and G. P. Paudel, “Security risks from climate change and environmental degradation : implications for sustainable land use transformation in the Global South,” Curr Opin Environ Sustain, no. June, pp. 1–11, 2023, https://doi.org/10.1016/j.cosust.2023.101322
G. Taylor and S. Vink, “Climate Risk Management Managing the risks of missing international climate targets,” Clim Risk Manag, vol. 34, no. October, p. 100379, 2021, https://doi.org/10.1016/j.crm.2021.100379
A. Raihan, M. I. Pavel, D. A. Muhtasim, S. Farhana, O. Faruk, and A. Paul, “The role of renewable energy use, technological innovation, and forest cover toward green development: Evidence from Indonesia,” Innovation and Green Development, vol. 2, no. 1, p. 100035, 2023, https://doi.org/10.1016/j.igd.2023.100035
A. Kiani et al., “A study on degradation and CO2 capture performance of aqueous amino acid salts for direct air capture applications,” Greenhouse Gases: Science and Technology, vol. 0, pp. 1–12, 2024, https://doi.org/10.1002/ghg.2302
A. Rehman, M. M. I. O. Alam, R. Alvarado, M. Murshed, C. Işık, and H. Ma, “Globalization and renewable energy use: how are they contributing to upsurge the CO2 emissions? A global perspective,” Environmental Science and Pollution Research, vol. 30, no. 4, pp. 9699–9712, 2023, https://doi.org/10.1007/s11356-022-22775-6
A. Zulys, F. Yulia, N. Muhadzib, and Nasruddin, “Biological Metal–Organic Frameworks (Bio-MOFs) for CO2 Capture,” Ind Eng Chem Res, vol. 60, no. 1, pp. 37–51, 2020, https://doi.org/10.1021/acs.iecr.0c04522
NOAA, “Trends in Atmospheric Carbon Dioxide.” [Online]. Available: https://gml.noaa.gov/ccgg/trends/global.html
F. I. Dinul, H. Nurdin, D. Rahmadiawan, Nasruddin, I. A. Laghari, and T. Elshaarani, “Comparison of NaOH and Na2CO3 as absorbents for CO2 absorption in carbon capture and storage technology,” Journal of Engineering Researcher and Lecturer, vol. 2, no. 1, pp. 28–34, Apr. 2023, https://doi.org/10.58712/jerel.v2i1.23
I. O. Ekemezie and W. N. Digitemie, “Carbon Capture and Utilization (CCU): A review of emerging applications and challenges,” Engineering Science & Technology Journal, vol. 5, no. 3, pp. 949–961, 2024, https://doi.org/10.51594/estj.v5i3.949
A. E. Ogungbenro, D. V. Quang, K. Al-Ali, and M. R. M. Abu-Zahra, “Activated Carbon from Date Seeds for CO2 Capture Applications,” Energy Procedia, vol. 114, pp. 2313–2321, 2017, https://doi.org/10.1016/j.egypro.2017.03.1370
N. Mokti, A. Borhan, S. N. A. Zaine, and H. F. M. Zaid, “Synthesis and Characterisation of Pyridinium-Based Ionic Liquid as Activating Agent in Rubber Seed Shell Activated Carbon Production for CO2Capture,” Journal of Advanced Research in Fluid Mechanics and Thermal Sciences, vol. 82, no. 1, pp. 85–95, 2021, https://doi.org/10.37934/arfmts.82.1.8595
T. A. Saleh, “Chapter 2 - Adsorption technology and surface science,” Interface Science and Technology, vol. 34, pp. 39–64, 2022, https://doi.org/10.1016/B978-0-12-849876-7.00006-3
M. S. B. Reddya, D. Ponnamma, K. K. Sadasivuni, B. Kumar, and A. M. Abdullah, “Carbon dioxide adsorption based on porous materials,” RSC Adv, vol. 11, no. 21, pp. 12658–12681, 2021, https://doi.org/10.1039/D0RA10902A
D. Liu, W. Zhang, and W. Huang, “Effect of removing silica in rice husk for the preparation of activated carbon for supercapacitor applications,” Chinese Chemical Letters, vol. 30, no. 6, pp. 1315–1319, 2019, https://doi.org/10.1016/j.cclet.2019.02.031
M. M. Sabzehmeidani, S. Mahnaee, M. Ghaedi, H. Heidari, and V. A. L. Roy, “Carbon based materials: a review of adsorbents for inorganic and organic compounds,” Mater Adv, vol. 2, pp. 598–627, 2021, https://doi.org/10.1039/D0MA00087F
E. Taer, A. Agustino, E. S. Gultom, and R. Taslim, “Sustainable development of biomass-derived activated carbon through chemical and physical activations and its effect on the physicochemical and electrochemical activity,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 45, no. 1, pp. 319–330, 2023, https://doi.org/10.1080/15567036.2023.2168319
A. D. Alsulaili, A. A. Refaie, and H. A. Garcia, “Adsorption capacity of activated carbon derived from date seeds: Characterization, optimization, kinetic and equilibrium studies,” Chemosphere, vol. 313, p. 137554, 2023, https://doi.org/10.1016/j.chemosphere.2022.137554
N. Khotimah, A. Martin, and E. Taer, “Pengaruh Temperatur Aktivasi Fisika Terhadap Daya Serap Iodium Karbon Aktif Berbahan Dasar Limbah Plastik Polyethylene Terephthalate (PET), [Effect of Physical Activation Temperature on Iodine Absorbency of Activated Carbon Based on Polyethylene Terephthalate Plastic Waste (PET)]” Turbo: Jurnal Program Studi Teknik Mesin, vol. 13, no. 01, pp. 134–141, 2024, https://doi.org/10.24127/trb.v13i1.3314
N. Khotimah, A. Martin, E. Taer, Nasruddin, and I. Taib, “Synthesis and Characterization of a Novel Microporous Material from Polyethylene Terephthalate Plastic Waste,” Journal of Advanced Research in Micro and Nano Engineering, vol. 33, no. 1, pp. 112–122, 2025, https://doi.org/10.37934/armne.33.1.112122
R. C. Bansal and M. Goyal, Activated Carbon Adsorption. CRC Press, 2005. https://doi.org/10.1201/9781420028812
J.-Y. Wang, E. Mangano, S. Brandani, and D. M. Ruthven, “A review of common practices in gravimetric and volumetric adsorption kinetic experiments,” Adsorption, vol. 27, pp. 295–318, 2021, https://doi.org/10.1007/s10450-020-00276-7
A. Martin et al., “High-Pressure Adsorption Isotherms of Carbon Dioxide and Methane on Activated Carbon From Low-Grade Coal of Indonesia High-Pressure Adsorption Isotherms of Carbon Dioxide and Methane on Activated Carbon From Low-Grade Coal of Indonesia,” Heat Transfer Engineering, vol. 38, no. 4, pp. 396–402, 2017, https://doi.org/10.1080/01457632.2016.1194702
E. Satar, P. Nyfeler, B. Bereiter, C. Pascale, B. Niederhauser, and M. Leuenberger, “Investigation of adsorption and desorption behavior of small-volume cylinders and its relevance for atmospheric trace gas analysis,” Atmos Meas Tech, vol. 13, no. 1, pp. 101–117, 2020, https://doi.org/10.5194/amt-13-101-2020, 2020
Nasruddin, A. Martin, M. I. Alhamid, and D. Tampubolon, “Adsorption Isotherms of Hydrogen on Granular Activated Carbon Derived From Coal and Derived From Coconut Shell,” Heat Transfer Engineering, vol. 38, no. 4, pp. 403–408, 2017, https://doi.org/10.1080/01457632.2016.1194703
S.-J. Han, S.-X. Sang, P.-P. Duan, J.-C. Zhang, W.-X. Xiang, and A. Xu, “The effect of the density difference between supercritical CO2 and supercritical CH4 on their adsorption capacities: an experimental study on anthracite in the Qinshui Basin,” Pet Sci, vol. 19, no. 4, pp. 1516–1526, 2022, https://doi.org/10.1016/j.petsci.2022.03.003
N. N. Nguyen, M. Galib, and A. V. Nguyen, “Critical Review on Gas Hydrate Formation at Solid Surfaces and in Confined Spaces—Why and How Does Interfacial Regime Matter?,” Energy & fuels, vol. 34, no. 6, pp. 6751–6760, 2020, https://doi.org/10.1021/acs.energyfuels.0c01291
S. Ntsondwa, V. Msomi, and M. Basitere, “Evaluation of the Adsorptive Process on Adsorbent Surfaces as a Function of Pressure in an Isosteric System Compared with Adsorption Isotherm,” ChemEngineering, vol. 6, no. 4, 2022, https://doi.org/10.3390/chemengineering6040052
S. Sultana, A. Pal, K. A. Rocky, A. Kowsar, I. M. Syed, and B. B. Saha, “Thermodynamic study of zeolite and graphene nanoplatelet-based composites for adsorption cooling systems,” Appl Therm Eng, vol. 254, p. 123850, 2024, https://doi.org/10.1016/j.applthermaleng.2024.123850
A. Chakraborty, B. B. Saha, S. Koyama, and K. C. Ng, “On the thermodynamic modeling of the isosteric heat of adsorption and comparison with experiments,” Appl Phys Lett, vol. 89, no. 17, p. 171901, 2006, https://doi.org/10.1063/1.2360925
A. Martin et al., “High-Pressure Adsorption Isotherms of Carbon Dioxide and Methane on Activated Carbon From Low-Grade Coal of Indonesia,” Heat Transfer Engineering, vol. 38, no. 4, pp. 396–402, 2017, https://doi.org/10.1080/01457632.2016.1194702
U. Thubsuang et al., “Efficient CO2 adsorption on porous carbon with nitrogen functionalities based on polybenzoxazine: High-pressure adsorption characteristics,” Appl Surf Sci, vol. 607, p. 155120, 2023, https://doi.org/10.1016/j.apsusc.2022.155120
B. Petrovic, M. Gorbounov, and S. M. Soltani, “Impact of Surface Functional Groups and Their Introduction Methods on the Mechanisms of CO2 Adsorption on Porous Carbonaceous Adsorbents,” Carbon Capture Science & Technology, vol. 3, p. 100045, 2022, https://doi.org/10.1016/j.ccst.2022.100045
P. A. S. Moura et al., “Assessing the potential of nanoporous carbon adsorbents from polyethylene terephthalate (PET) to separate ¬ CO2 from flue gas,” Adsorption, 2018, https://doi.org/10.1007/s10450-018-9943-4
X. Yuan, S. Li, S. Jeon, S. Deng, L. Zhao, and K. B. Lee, “Valorization of waste polyethylene terephthalate plastic into N-doped microporous carbon for CO2 capture through a one-pot synthesis,” J Hazard Mater, vol. 399, p. 123010, 2020, https://doi.org/10.1016/j.jhazmat.2020.123010
S. Li et al., “Diamond in the rough: Polishing waste polyethylene terephthalate into activated carbon for CO2 capture,” Science of the Total Environment, vol. 834, p. 155262, 2022, https://doi.org/10.1016/j.scitotenv.2022.155262
J. Wang et al., “Waste polyethylene terephthalate (PET) plastics-derived activated carbon for CO2 capture: a route to a closed carbon loop,” Green Chemistry, vol. 22, pp. 6836–6845, 2020, https://doi.org/10.1039/D0GC01613F
B. Kaur, R. K. Gupta, and H. Bhunia, “Chemically activated nanoporous carbon adsorbents from waste plastic for CO2 capture: Breakthrough adsorption study,” Microporous and Mesoporous Materials, vol. 282, pp. 146–158, 2019, https://doi.org/10.1016/j.micromeso.2019.03.025
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Awaludin Martin, Erman Taer, Nasruddin Nasruddin, Nur Khotimah

This work is licensed under a Creative Commons Attribution 4.0 International License.